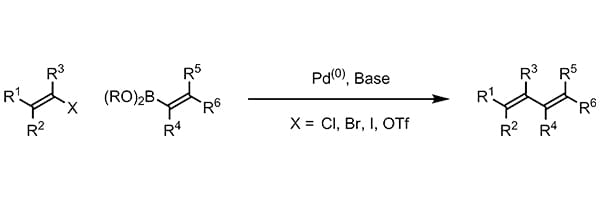
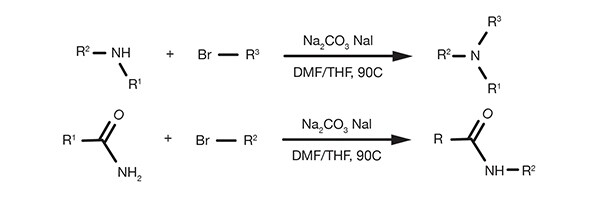
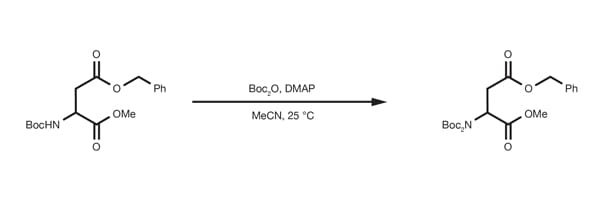
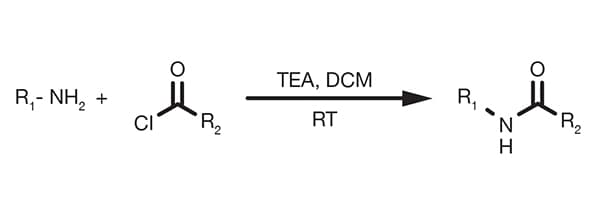
Popular Organic Reactions - find what you need easily
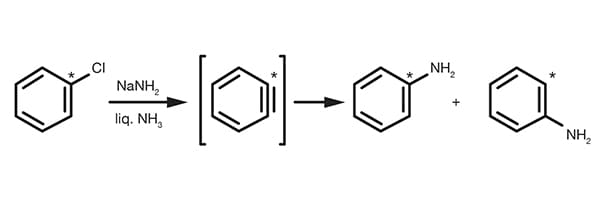
To help you run some of the most important reactions in synthetic organic chemistry a collection of practical reference information is provided. Each section contains some fundamental description, reference reaction protocols, literature references and examples.
For your convenience, all the chemicals you need to setup and run each reaction are listed, making it so easy to find them that you can get from the web to the lab in 5 minutes!