Aromatic Nucleophilic Substitution
The aromatic nucleophilic substitution is an extremely useful tool for the functionalisation of aryl compounds. It has been around since the early 50’s and is still very common in the organic synthesis practice, although more modern methods to couple nitrogen and aromatic carbon, such as the Buchwald-Hartwig cross-coupling (LINK TO SECTION), has been getting popularity in the last decade.
As all substitution reaction, a nucleophile, such as an amine or an alcohol, displaces a leaving group on the aromatic ring, this being usually a halide.
There are several different mechanisms associated to the aromatic nucleophilic substitution. Only three have practical relevance to organic synthesis:
- Addition-elimination mechanism
- Benzyne mechanism
- Aromatic SN1 of diazonium salts
The SNAr via addition-elimination is the most common reaction, occurring in the case of most substituted aryl halides. The electron withdrawing effect of the substituent facilitates the addition of the nucleophile on the ring by stabilising the negatively charged intermediates before the elimination of the leaving group.

The Benzyne mechanisms is relevant to unsubstituted aryl halides and proceeds via a benzyne intermediate as per the below scheme.

Finally the Aromatic SN1 occurs in the case of aromatic diazonium salts via the formation of an aryl cation intermediate by elimination of N2 that is subsequently submitted to addition of the nucleophile to give the substitution product.
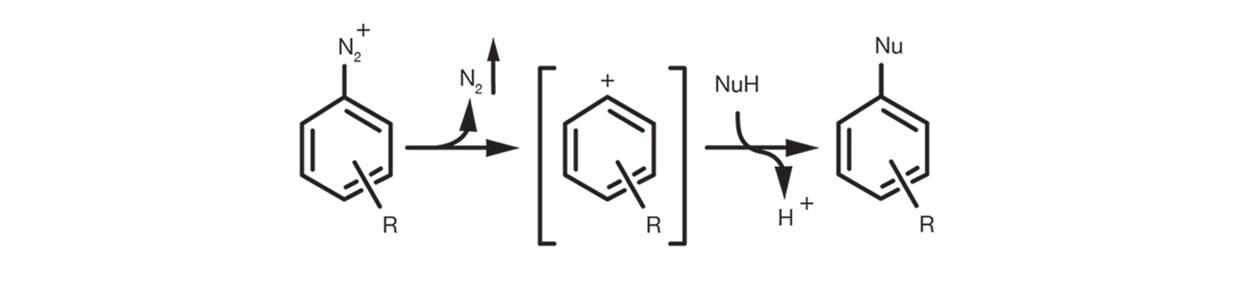
The reaction setup is quite simple. It is usually carried out in relatively polar organic solvents, protic and aprotic, such as DMSO, ethanol, DMF, NMP. It is supported by the use of an auxiliary base (tertiary amines and carbonates are the most common) and rarely needs significant excesses of nucleophile.
Reference Reaction Protocols
1.
Weight equimolar amounts of aryl halide and amine (5-50mmol). Add 1.5-2 equivalents of K2CO3 (or alternative base) in ethanol. Heat to reflux for 8-24h.
Aniline derivatives can usually be easily precipitated by pH shift.
2.
Weight equimolar amounts of aryl halide and alcohol and 1.5 equivalents, dissolve in THF and suspend 1.5 equivalents of NaH. Stir for 8-24h. The reaction can proceed at room temperature, however it is often necessary to heat up to 80-100°C.
Ether products are usually separated by extraction with water, polar or apolar solvents. MgSO4 is commonly used to remove water and vacuum to remove volatiles.
Thiols can be effectively used as nucleophile and the reaction protocol is typically the same as for the alcohols.
3.
Dissolve the aryl halide, a slight excess of KCN (1.1-1.3 equivalents) and an equimolar amount of trimethylamine in DMSO. Stir the reaction at about 50°C for 12-24h adding water dropwise.
Reaction products can be diluted in water and extracted with EtOAc, or other aprotic polar solvents.
Chromatographic purification is often necessary.
Examples
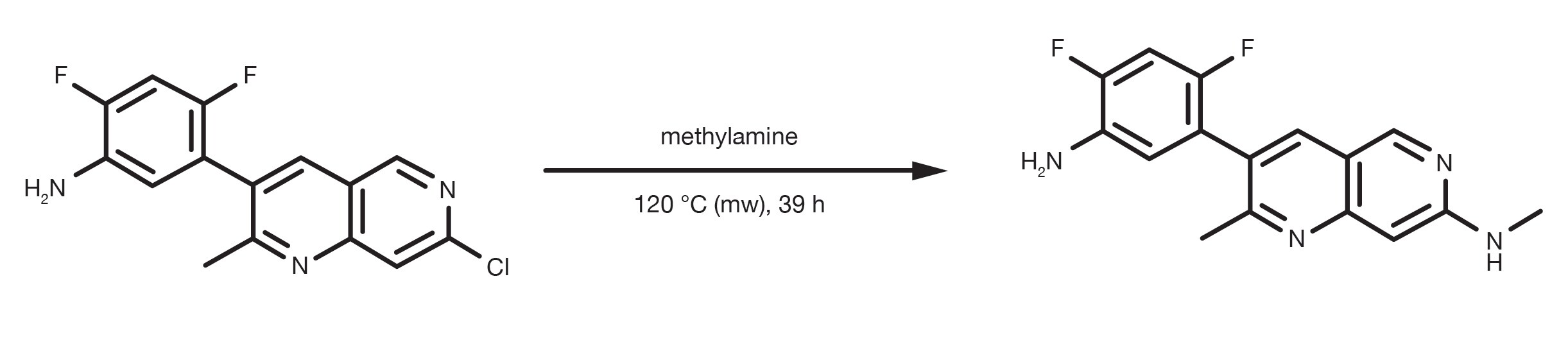
Patent Reference: WO2013134298

Patent Reference: WO2014149164
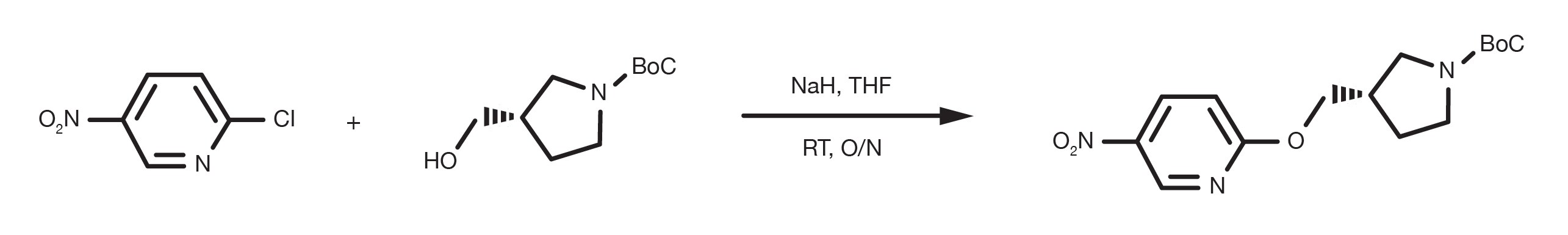
Patent Reference: WO2016012477
Key literature references
- Chem. Rev., 1951, 49 (2): 273–412. https://doi.org/10.1021/cr60153a002
- Molecules 2006, 11, 130-133. https://doi.org/10.3390/11020130
Product Selection
Solvents:
DMSO
EtOH
DMF
N-Me pyrrolidone (NMP)
Iso-Propanol
AcN
n-butanol
propanol
dioxane
Basic ingredients / Additives:
Bases:
Triethylamine
N,N-Diisopropylethylamine
CsF
Methylamine
NaH
KOH
CsCO3
NaOMe
t-BuONa
1,4-diazabicyclo[2.2.2]octane (DABCO)
MgSO4 (for workup)
Building blocks:
(poly)substituted aryl halides: F, Cl, Br.
Other substituents: Nitro, OH, amine, alkyl. Presence of electron withdrawal group preferred
N-Heteroaryl halides (-Cl are the most important ones)
Variously substituted pyridinyl derivatives are very reactive
(hetero)aryl diazonium salts
Nucleophiles:
amines (primary, secondary and tertiary)
aliphatic alcohols (mostly primary and secondary)
NH4OH
aromatic alcohols (variously substituted phenols)
aliphatic thiols
aromatic thiols
NaCN
KCN
NaN3
Catalysts:
Silica gel can be used as catalyst in some high temperature reactions of unsubstituted chlorobenzene
