Amide Synthesis
Amination of acyl chlorides or anhydrides
The direct coupling between a carboxylic acid and an amine is competing with the acid/base proton exchange and it hardly is a suitable choice in synthetic chemistry. The most common strategy is the conversion of the acid to an activated form (i.e. more electrophilic), such as the acyl chloride or the anhydride. These species readily react with primary and secondary amines to give the corresponding amide.

Acylation of amine by acyl chloride is often referred to as Schotten–Baumann reaction, from the names of its inventors.
Most commonly, the reaction proceeds rapidly at room temperature in aprotic solvents in the presence of a suitable base, such as a tertiary amine or pyridine. Often an aqueous solution of the base is added drop-wise, eventually generating a biphasic system.
The Schotten-Baumann reaction and the coupling between the amine and an anhydride are mechanistically related, the only significant difference being the acid by-product: HCl in one case, a carboxylic acid in the other. They both require a base to drive the equilibrium to the right.
The preparation of the acyl chloride can be done in situ by mixing the carboxylic acid with thionyl chloride or oxalyl chloride in aprotic solvents, such as DCM, THF, or EtOAc. Refluxing the mixture for a few hours gives typically good conversions. It is necessary to isolate the acyl chloride before the amidation step with the amine.
Peptide chemistry derived methods (active ester intermediates)
Significant progresses in the synthesis of amides have been achieved in the last three decades by the research in the field of peptide synthesis. The big variety of peptide coupling reagents that are commercially available nowadays share a fundamental chemical principle: the synthesis of a highly activated ester. Although many of the peptide coupling reagents have been specifically designed for automated solid phase, or solution peptide synthesis, the same synthetic strategies can be used in principle for the synthesis of any amide. The most common reagents belong to two main groups:
- Carbodiimmides
- Hydroxybenzotriazole Aminium/Uronium or Phosphonium salts
Carbodiimide activation
The two most common reagents of this group are dicyclohexylcarbodiimide (DCC), diisopropylcarbodiimide (DIC) and 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). They react with carbonylic acids to form a highly reactive O-acylisourea that can be converted to amide by reaction with an amine with high yields and short reaction times.
The synthesis of O-acylisourea benefits from the use of apolar solvents when possible (e.g DCM), although many polar aprotic solvent can be used.
O-acylisoureas are among the most active intermediates for amide coupling however they can racemise spontaneously. This must be taken into consideration in the synthetic strategy.
This is also the reason why in peptide synthesis the activation of the carboxylic acid is usually done in the presence of hydroxybenzotriazoles additives, that react rapidly with the activated ester. In this case the activation step should be better described as a two step DCC/Hydroxybenzotriazole activation.
This second reaction step generates another activated ester that does not racemise but retain a sufficient level of activation for an efficient peptide coupling.
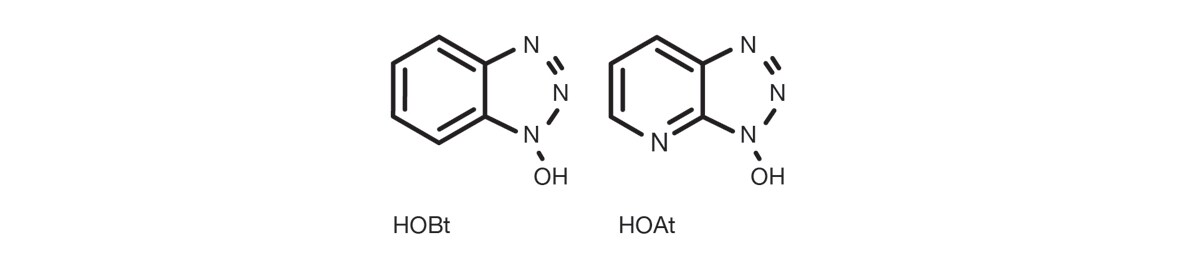
The most common triazoles for this applications are 1-hydroxy-benzotriazole (HOBt), and 1-hydroxy-7-aza-benzotriazole (HOAt).
Activation with Amonium/Uronium and Phosphonium salts of Hydroxybenzotriazole
A strategy to avoid the two-step acyl activation process in peptide chemistry is made possible by the use of ammonium/uronium or phosphonium salts of the hydroxytriazoles mentioned above. This way it is possible to avoid completely the use of DCC and the formation of the O-acylisourea intermediate.
HOBt has a corresponding uranium salt called HBTU and its common phosphonium salts are called BOP and PyBOP. The corresponding uronium and phosphonium salts of HOAt are HATU and AOP/PyAOP respectively.
HOBt – HBTU (or TBTU) – BOP or PyBOP
HOAt – HATU – AOP or PyAOP
The direct reaction between the carboxylic acid and HATU/AOP/PyAOP or HBTU/BOP/PyBOP happens in the presence of a base (usually DIEA, diisopropylethylamine) in polar aprotic organic solvents, such as DMF, or AcN.
These activated esters can be efficiently coupled to an amine using reaction conditions similar to the Schotten–Baumann reaction (base, aprotic solvent, RT).
Reference Reaction Protocols
Schotten Baumann reaction:
1.
Prepare a stirring solution of the amine in DCM add an equimolar amount of base (e.g DIEA), followed by 1-to-2 equivalents of the acid chloride. Stir at RT for 8-16 h. Typically the reaction is quenched with H2O and extracted with DCM or other organic solvents depending on the characteristics of the amide.
2.
Prepare the solution of the amine and base (equimolar) at 0°C. Cool the mixture down under stirring conditions to -75°C, then add the acyl chloride. Stir for 1-6h, then quench with H2O, extract with DCM and proceed to the product purification.
Carbodiimmide method:
Prepare a solution of the acid in DMF adding about 2 or 3 equivalents of base. Col the solution down to 0°C and add two equivalents of DCC or EDC. Stir at RT for 30-60min, then dilute with H2O and extract with an organic solvent according to the amide product’s properties. Proceed to the product purification.
HATU/HBTU
Prepare a solution of the acid in DMF at 0°C then add two equivalents of HATU (550 mg, 1.4 mmol) and 3 equivalents of base (e.g. DIEA, or TEA). Finally add a slight excess of the amine (compared to the acid) and stir at RT for 30-60min. Dilute with water and extract with an organic solvent according to the amide product’s properties. Proceed to the product purification.
The reference protocol remains the same in case of use of different activators, such as AOP, PyBOP, BOP, etc.
In case of use of HBTU the protocol remains similar, however it is often necessary to increase the temperature of the reaction and some authors report reflux conditions and conversion times slightly longer, up to 2 or 3 hours.
Combined use of Carbodiimmide and hydroxybenzotreiazole
Prepare a solution of the acid (700 mg, 3.2 mmol) in DMF (10 mL), add 3 equivalents of base (e.g. DIEA, NH4Cl, or TEA) while cooling the reaction to 0°C. Treat the mixture with 2 equivalents of ethylcarbodiimide (or other carbodiimmide) and 2 equivalents of HOBt (or HOAt), finally add 1.5 equivalents of the amine. Stir at RT for 30-60 min. Quench with H2O and extract with an organic solvent according to the amide product’s properties. Proceed to the product purification.
Examples

Patent Reference: WO2015191681

Patent Reference: WO2015129926

Patent Reference: WO2015140133

Patent Reference: WO2014149164

Patent Reference: WO2015129926
Key literature references
- Schotten, C. Berichte der deutschen chemischen Gesellschaft. 1884, 17, 2544. doi:10.1002/cber.188401702178.
- Baumann, E. Berichte der deutschen chemischen Gesellschaft. 1886, 19, 3218. doi:10.1002/cber.188601902348.
- Emil Fischer Berichte der deutschen chemischen Gesellschaft. 1903, 36, 2982–2992. doi:10.1002/cber.19030360356.
- Jaradat, Da’san M. M. "Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation". Amino Acids. 2017, 50 (1): 39–68. doi:10.1007/s00726-017-2516-0. ISSN 0939-4451
- El-Faham A, Albericio F. "Peptide coupling reagents, more than a letter soup". Chemical Reviews. 2011, 111, 6557–602. doi:10.1021/cr100048w
Product Selection
Solvents:
DCM
THF
DMF
EtOAc
iProOAc
AcN
Work-up:
MeOH
EtOH
iProOH
hexane
heptane
Basic ingredients / Additives:
Diisopropylethylamine
Triethylamine
Pyridine
H2SO4
NH2OH
Work-up:
MgSO4
Na2SO4
LiCl
t-NaHCO3
Silica gel
Building blocks:
Alkyl or (het)aromatic acyl chlorides (diversity is important here. Beware of need for protecting groups in case of multiple functionalities, particularly amino-derivatives)
Alkyl or (het)aromatic carboxylic acids (see above for diversity and functionalisation. These are to be used with the in situ activation (acyl chloride or peptide chemistry activation methods))
Minor importance:
Alkyl nitriles (amide from nitrile hydrolysis)
Saturated cyclic ketones (for Sheppard amidation to give lactams)
Aryl alkyl ketones (with alkyl chain up to 5 carbons)
Activating agents:
Thionyl chloride
Oxalyl chloride
Dicyclohexylcarbodiimide (DCC)
Diisopropylcarbodiimide (DIC)
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)
1-hydroxy-benzotriazole (HOBt)
1-hydroxy-7-aza-benzotriazole (HOAt)
1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU)
N,N,N',N'-Tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU)
(Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP)
(Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyBOP)
Tris(dimethylamino)(3H-1,2,3-triazolo[4,5-b]pyridin-3-yloxy)phosphorus hexafluorophosphate (AOP)
(7-Azabenzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyAOP)
Tris(2,2,2-trifluoroethyl) borate
